
Course focused on classical and advanced synthetic strategies, as well as analytical and spectroscopic techniques, for the design, synthesis, and physicochemical characterization of established drugs and novel biologically active compounds.
- Teacher: Donatella Boschi
- Teacher: Agnese Chiara Pippione
- Teacher: Konstantin Chegaev
- Teacher: Maela Manzoli

- Teacher: Donatella Boschi
- Teacher: Eleonora Gianquinto
- Teacher: Katia Martina
- Teacher: Francesca Spyrakis

- Teacher: Gianluca Miglio

- Teacher: Donatella Boschi
- Teacher: Katia Martina
- Teacher: Agnese Chiara Pippione

The aim of the course is describing the preclinical regulatory approach before clinical development and marketing authorization of medicinal product.
- Teacher: Donatella Boschi
- Teacher: Jan Arseen Rosier

- Teacher: Katia Martina

- Teacher: Donatella Boschi
- Teacher: Katia Martina
- Teacher: Agnese Chiara Pippione

The course proposes knowledges on botanicals, their taxonomic classification and the correlation of their health properties with the chemical composition on the basis of scientific evidence.
Basis of nutrition and dietetics will also be addressed, which underlie the rationale for the formulation of functional/functionalized foods, food supplements, and foods intended for specific groups of the population.
Basis of nutrition and dietetics will also be addressed, which underlie the rationale for the formulation of functional/functionalized foods, food supplements, and foods intended for specific groups of the population.
- Teacher: Cecilia Lucia Cagliero
- Teacher: Erica Liberto
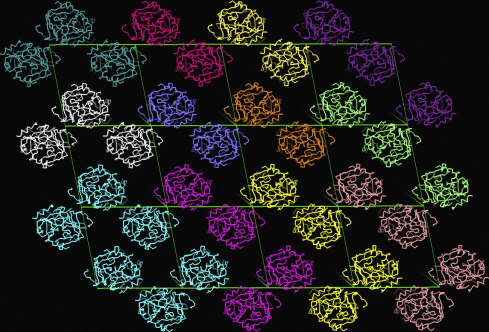
The aim of this course is to provide an outline of the basic principles and methods of protein crystallography, learning how crystallographic data can contribute today to the different phases of pharmaceutical research. The objective of this course is to show how the different stages of drug discovery research can benefit from the already available structural knowledge; to point out how experimental structure determination by X-ray analysis can be integrated into the hit/lead finding, triaging, validation and optimization phases inside Structure-based drug design (SBDD). It will emphasize the strengths, usefulness and application of protein crystallography, so that any medicinal chemist engaging in a new research program and having access to a structural biology group, can gauge if, and how, his project could potentially benefit from this technology. Medicinal chemists, in particular those working in the industry, have access to large, public as well as proprietary, depositories of refined crystal structures. To make proper use of these data, it is essential for them to be well aware of the limitations and potential uncertainties associated with X-ray.
- Teacher: Donatella Boschi
- Teacher: Osman Asghar Mirza
- Teacher: Cecilia Lucia Cagliero
- Teacher: Erica Liberto
